The Pharmaceutical Cannabis Report: 2nd Edition
Research on the endocannabinoid system is one of the most promising avenues for new therapeutics in medicine at the moment. However, regulations are constantly shifting. This report has been compiled to assist interested parties in understanding the market for the medical use of cannabinoids and where it may be heading. In both Europe and the United States, the sales of unapproved cannabis products such as smokable flower and full spectrum oils dwarfs those of approved pharmaceuticals. In Europe however, there is a clear trend towards the use of more precise formulations of unapproved cannabis products as well as pharmaceutical products. In the US, sales of medical flower and vaporised products remain dominant amongst unapproved medicines, but there is also an increase in sales of pharmaceuticals. Prohibition Partners has identified at least 30 late-stage clinical trials using cannabinoid therapeutics, any of which will probably have a large impact on the medicinal cannabis space. In the medium to long term, Prohibition Partners expect to see the development of a range of new cannabinoid therapeutics approved across the globe. Operators in the space can expect to see these products gradually taking up more of the market share at the expense of unapproved flower and full spectrum oils over the next 10 years.
The Pharmaceutical Cannabis Report: 2nd Edition
Research on the endocannabinoid system is one of the most promising avenues for new therapeutics in medicine at the moment. However, regulations are constantly shifting. This report has been compiled to assist interested parties in understanding the market for the medical use of cannabinoids and where it may be heading. In both Europe and the United States, the sales of unapproved cannabis products such as smokable flower and full spectrum oils dwarfs those of approved pharmaceuticals. In Europe however, there is a clear trend towards the use of more precise formulations of unapproved cannabis products as well as pharmaceutical products. In the US, sales of medical flower and vaporised products remain dominant amongst unapproved medicines, but there is also an increase in sales of pharmaceuticals. Prohibition Partners has identified at least 30 late-stage clinical trials using cannabinoid therapeutics, any of which will probably have a large impact on the medicinal cannabis space. In the medium to long term, Prohibition Partners expect to see the development of a range of new cannabinoid therapeutics approved across the globe. Operators in the space can expect to see these products gradually taking up more of the market share at the expense of unapproved flower and full spectrum oils over the next 10 years.
Report Only
-£800 + VAT
Report + Market Sizing
-£1500 + VAT
Report + Market Sizing + Clinical Trials Database
-£2000 + VAT
PARTNERS
OVERVIEW
KEY FINDINGS
Data Packages
Key Findings
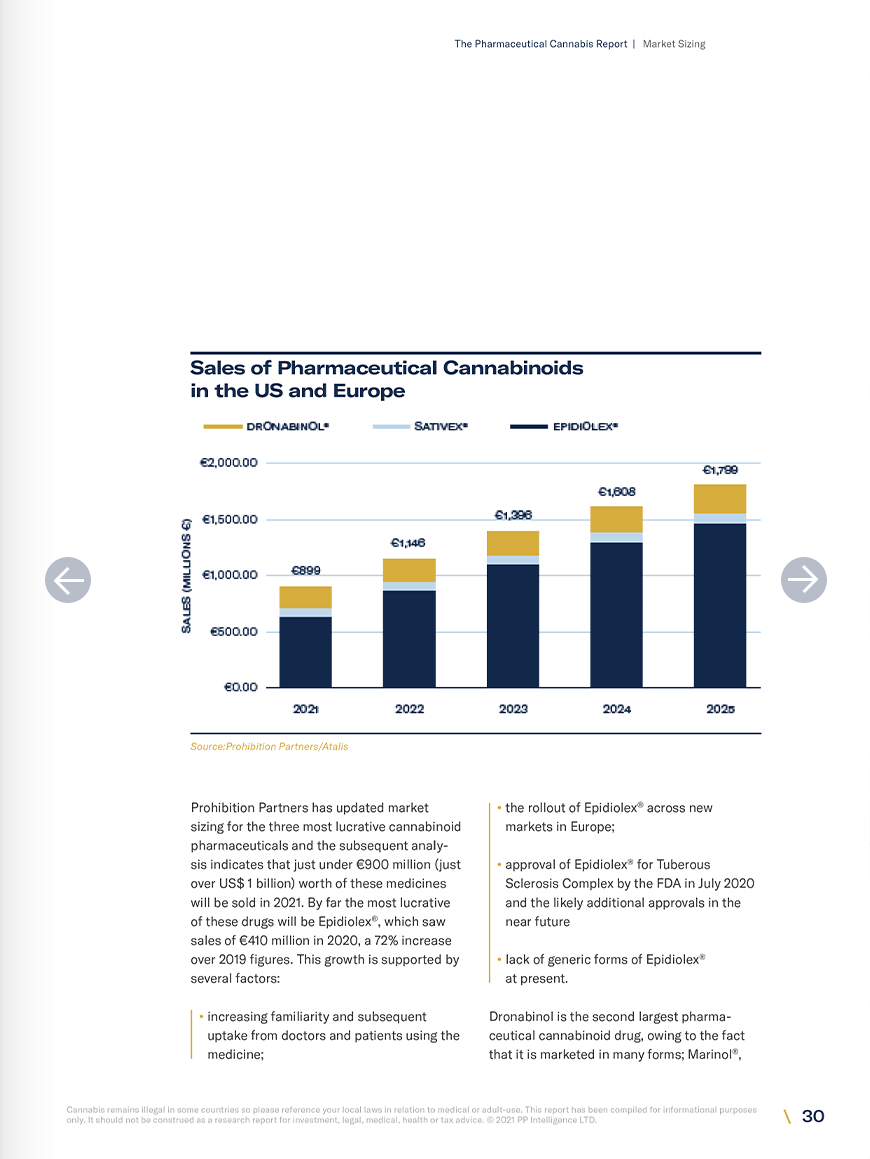
- Prohibition Partners estimate the market size for the three most widely used cannabinoid pharmaceuticals (Epidiolex ®, Sativex ®, dronabinol) to be worth just under €900 million as of 2021, growing to €1.8 billion by 2025.
- Prohibition Partners’ global surveys of cannabis users indicates that around 40% of users have some medical component to their usage.
- The current global spend on pain medications worldwide is estimated at US$63-85 billion each year. However, no single cannabinoid drug has received widespread approval in the treatment of pain.
Definition and Scope
- Definitions
- Methodology
- Market Sizing
- Market Research
Contents Overview
- Definitions
- Introduction
- Case Study: GW Pharmaceuticals
- Clinical Trials Review
- Market Sizing
- Patents
- New Formulations & Devices
- Minor Cannabinoids
- Synthetic Cannabinoids
- Expert Interviews
- Abbreviations
Market Sizing
- Estimated Sales of Major Pharmaceutical Cannabis Products
- Market Sizing Methodology
Clinical Trials Review
- Trends in Clinical Trials of Cannabinoids
- Analysis of Key Areas of Research
- Key Ongoing Clinical Trials
Case Study
- GW Pharmaceuticals Case Study
- What it Takes to Bring a Cannabinoid Drug to Market
- A Timeline of GW Pharmaceuticals' Development
- Financial Analysis of GW Pharmaceuticals (over 15 years)
- GW Pharmaceuticals' Focus on Research & Development
Patents
- Current Trends in Cannabis Patents
- Life Cycle Management and 'The Patent Cliff'
- Generic Cannabinoid Opportunities
- GW vs. Canopy Growth: A Looming Patent War?
New Formulations & Devices
- Routes of Administration for Cannabinoids
- Innovative Delivery Formats
Minor Cannabinoids
- Overview of Most Prominent Minor Cannabinoids
Synthetic Cannabinoids
- Pros and Cons of Synthetic Cannabinoids
- Estimated Production Cost for Cannabinoid Production Methods
- Current State of Play in the Synthetic Cannabinoid Space
Expert Interviews
- Dr. Peter Grinspoon, Harvard Medical School
- Shaun Jarvis, PureForm Global
As Trusted By
Insights
on the global cannabis industry
International Cannabis Weekly newsletter brings you the most important developments, news and informed commentary on the global cannabis industry. Join our community of 80,000+ subscribers.